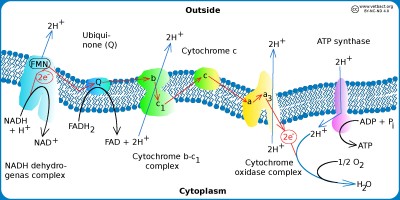
Electron transport chain
The figure shows a schematic view of the electron transpor chain in bacteria with aerobic metabolism. Hydrogen ions (protons) are pumped out (blue arrows) out of the cell by using three different enzyme complexes and a proton gradient is formed over the plasma membrane. The proton gradient is the energy (proton motive force) used by the ATP synthase to produce ATP. The electrons jump gradually between the various components of the enzyme complexes, which have increased electronegativity from left to right in the figure. The higher the electronegativity, the stronger the attraction of electrons. Oxygen is the component of the system that has the highest electronegativite and it is also the final electron acceptor.
Image: Karl-Erik Johansson (BVF, SLU) - Click on the image to enlarge it.
Introduction
During the glycolysis, two molecules of pyruvate, each having 3 carbon atoms, are formed from a glucose molecule having 6 carbon atoms. Under aerobic conditions and if the current bacterium has aerobic metabolism, the pyruvate molecule is not fermented, but can instead be converted to acetyl-Coenzyme A (actyl-CoA). The acetyl group in acetyl-CoA has two carbon atoms since carbon dioxide (CO2) has been cleaved off and can now enter the Krebs cycle (= citric acid cycle), as an acetyl group. The Krebs cycle is described in a separate section and is a precursor to the electron transport chain (= respiration chain).
The electron transport chain
All enzymes required for the electron transport chain of bacteria are membrane bound as in eukaryotic cells, but in bacteria these molecules are present in the plasma membrane because bacteria have no mitochondria. The hydrogen ion gradient, which drives ATP synthesis, is thus generated across the plasma membrane. The electron transport chain consists of a series of enzyme complexes, which incrementally take care of the electrons that are formed when NADH and FADH2 (from the Krebs cycle) are oxidized to NAD+ and and FAD, respectively. At the same time, hydrogen ions (protons) are pumped out of the bacterial cell. When these hydrogen ions then pass to another membrane-bound enzyme complex (ATP synthase) on their way back into the cytoplasm, ATP is generated while the electrons are finally taken care of by oxygen and water is formed with the hydrogen ions present in the cytoplasm.
Variants of the electron transport chain
There are various variants of the electron transport chain and Escherichia coli, for instance, lacks cytochrome C oxidase as do most other bacteria in the family Enterobacteriaceae. These bacteria have instead a terminal cytochrome bo3 oxidase. Some bacteria have enzyme systems for anaerobic respiration, where molecules other than oxygen form the terminal electron acceptor. The following molecules may be used: nitrate, nitrite, ferric iron (Fe3+), sulfate, carbon dioxide, and small organic molecules such as fumarate. In anaerobic respiration, we talk about terminal reductases instead of terminal oxidases.