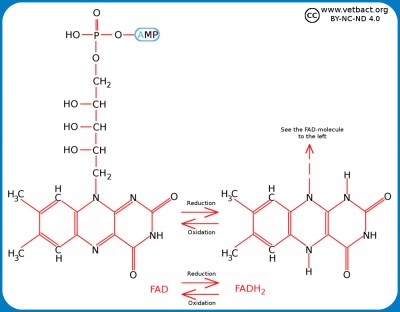
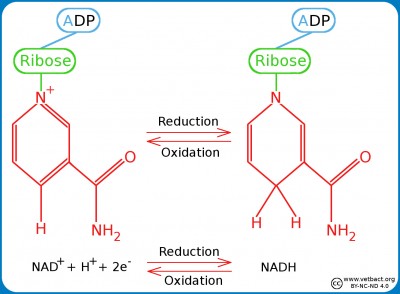
NAD and FADThe picture shows the structure changes of FAD during oxidation/reduction. Note the two extra hydrogen atoms in the reduced form of FAD. The chemical structure of AMP and ribose are shown in the picture under ATP in the term list. - Click on the image to enlarge it. The picture shows the structure changes of NAD during oxidation/reduction. Note the extra hydrogen atom in the reduced form of NAD. The chemical structure of ADP and ribose are shown in the picture under ATP in the term list. - Click on the image to enlarge it. Nicotinamide adenine dinucleotide (NAD)NAD is a dinucleotide consisting of an adenine moiety and a nicotinamide moiety which are joined by two phosphate groups between the ribose moieties of the respective nucleotide. NAD is present in an oxidized form (NAD+) and a reduced form (NADH) in all living cells and this is essential for all living organisms. FunctionsNAD participates in so-called redox processes in the cell, i.e. chemical reactions where one molecule is reduced and another molecule is oxidized. NAD+ is thus the oxidized form of NAD, which can oxidize other molecules and then itself be reduced to NADH by cathing electrons (e-). NADH can in turn reduce other molecules by emitting electrons. The most important function of NAD are these electron transferring reactions. NAD also has other functions, e.g. as substrates for enzymes, which catalyze modification of macromolecules ("post translational modification") by cleavage or binding of chemical groups to e.g. proteins. OtherThe so-called V-factor, which is needed for certain bacteria to grow in artificial media, is in fact nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate (NADP, see below).
|